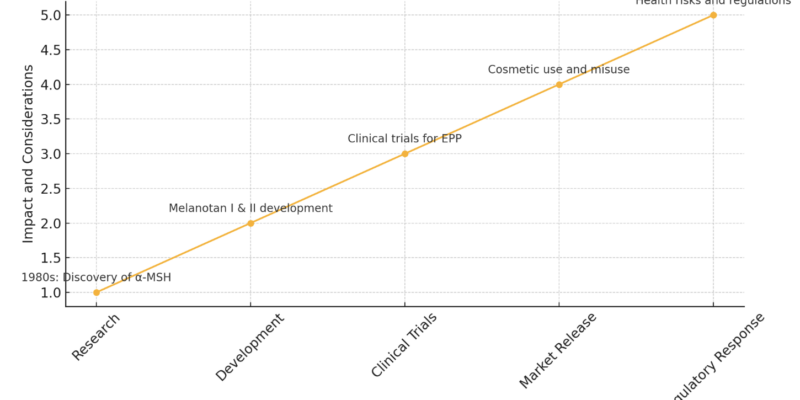
The Evolution of Melanotan
The Development of Melanotan: A Journey from Research to Application
The pursuit of a perfect tan has led to various methods over the years, from sunbathing to tanning salons and self-tanning products. However, these methods often come with risks such as skin damage and cancer from UV exposure. Enter Melanotan, a synthetic peptide developed to offer a safer alternative by stimulating the production of melanin, the pigment responsible for skin colour. This blog delves into the development of Melanotan, from its inception in scientific research to its controversial status in the market today.
The Origins of Melanotan
Early Research and Discovery
The story of Melanotan begins in the 1980s at the University of Arizona, where researchers were exploring ways to reduce skin cancer rates. Dr. Victor Hruby and his team were particularly interested in the hormone alpha-melanocyte-stimulating hormone (α-MSH), which naturally occurs in the body and is responsible for stimulating melanocytes, the cells that produce melanin.
Melanin not only gives skin its color but also provides protection against the harmful effects of ultraviolet (UV) radiation. The idea was that by increasing melanin production, people could achieve a tan without the need for UV exposure, thereby reducing the risk of skin cancer.
Development of Melanotan I and II
The initial compound developed was known as Melanotan I (afamelanotide). This peptide was designed to mimic the effects of α-MSH and stimulate melanin production. Early studies showed promising results in terms of inducing tanning without sun exposure. However, the research also indicated that the compound had a relatively short half-life in the body, meaning it broke down quickly and required frequent administration.
To address this issue, researchers developed Melanotan II, a more stable and potent variant. Melanotan II not only induced tanning more effectively but also had a longer duration of action. Interestingly, during clinical trials, researchers discovered that Melanotan II had an unexpected side effect: it significantly increased libido. This led to further investigations and the eventual development of bremelanotide, a related compound used to treat sexual dysfunction.
Mechanism of Action
Melanotan works by binding to melanocortin receptors in the body, which are involved in various physiological processes including pigmentation, inflammation, and energy homeostasis. The primary target of Melanotan is the melanocortin 1 receptor (MC1R) found on melanocytes. Activation of MC1R leads to an increase in the production of eumelanin, the dark pigment that provides UV protection.
Melanotan I (Afamelanotide)
Afamelanotide is designed to be administered via subcutaneous implants, providing a controlled release of the peptide over several weeks. This long-acting formulation allows for a sustained increase in melanin production, making it suitable for patients with conditions like erythropoietic protoporphyria (EPP), a rare genetic disorder that causes extreme sensitivity to sunlight.
Melanotan II
Melanotan II is typically administered via injection and has a shorter duration of action compared to afamelanotide. Its ability to induce tanning, increase libido, and reduce appetite has made it popular among bodybuilders and individuals seeking cosmetic tanning. However, the non-medical use of Melanotan II raises significant safety and ethical concerns.
Clinical Development and Applications
Afamelanotide and EPP
The clinical development of afamelanotide focused on its potential to treat EPP. Clinical trials demonstrated that afamelanotide could significantly reduce the pain and phototoxic reactions experienced by EPP patients when exposed to sunlight. In 2014, the European Medicines Agency (EMA) approved afamelanotide (Scenesse) for the treatment of EPP, making it the first melanocortin receptor agonist to receive regulatory approval for a medical condition.
Potential for Other Conditions
Beyond EPP, researchers have explored the use of afamelanotide and other melanocortin agonists for conditions such as vitiligo, a disorder characterised by loss of skin pigmentation, and polymorphous light eruption (PLE), a form of sun allergy. While the results have been mixed, the potential for melanocortin-based therapies to address a variety of dermatological and systemic conditions remains an area of active investigation.
Conclusion
The development of Melanotan represents a fascinating journey from the laboratory to the market, highlighting both the promise and peril of new medical technologies. While afamelanotide offers significant benefits for patients with specific conditions, the misuse of Melanotan II underscores the need for careful regulation and public education. As we continue to explore the potential of melanocortin-based therapies, it is vital to ensure that these innovations are used safely and ethically, prioritising the well-being of individuals over the pursuit of cosmetic ideals.
Here’s an illustration highlighting the evolution of Melanotan, from its initial research phase to market release and regulatory response. The graph shows the key stages and impacts associated with its development, along with annotations for each major milestone. This visual aid helps to summarise the journey of Melanotan, emphasising both its medical potential and the challenges it faces in the market





This Post Has 0 Comments